Part:BBa_K4345008
NarX H399-E
The mutant was designed based on a study of Cavicchioli et al., (1995). The histidine on place 399 was replaced by glutamic acid. This allows the NarX mutants to dimerize but blocks it from phosphorylating NarL (the second messenger).
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 659
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 659
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 260
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 659
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 659
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
This particular narX protein was derived from E. coli.
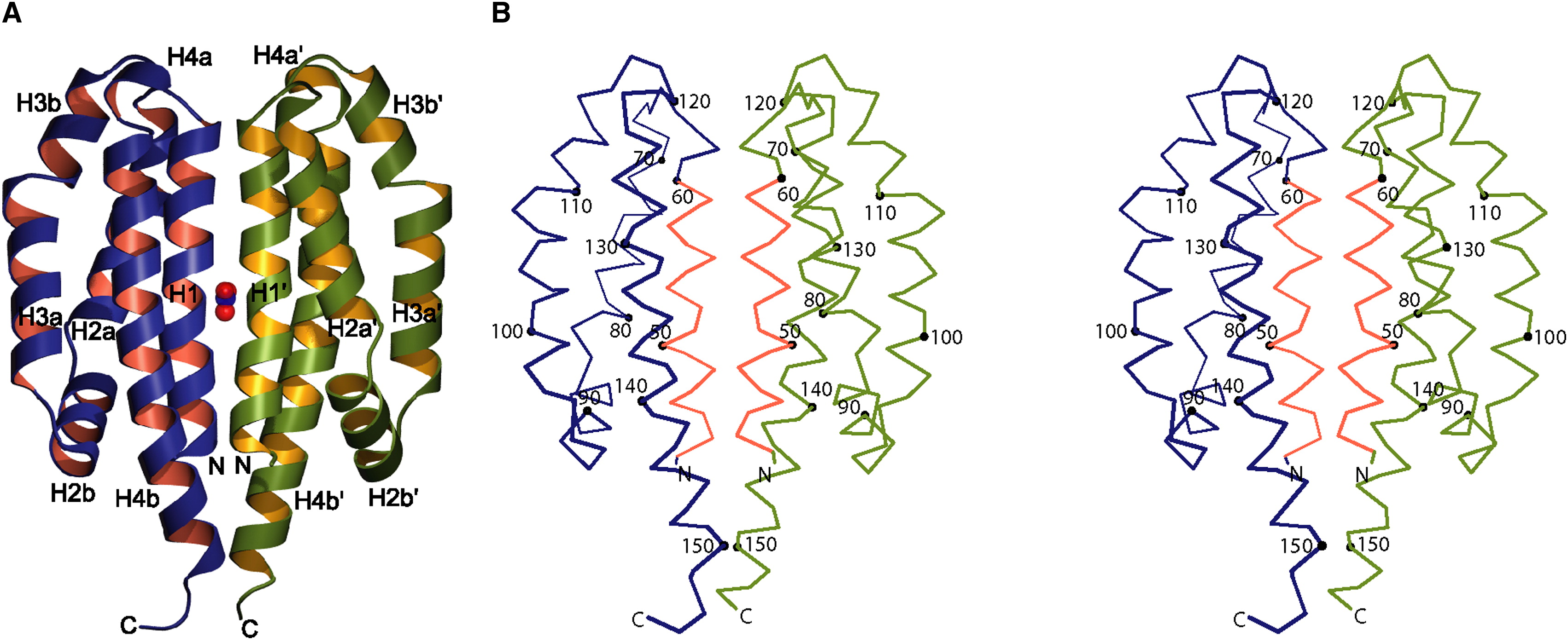
Image obtained from Cheung & Hendrickson, 2009
References
Cavicchioli, R., Schröder, I., Schröder, S., Constanti, M., & Gunsalus, R. P. (1995). The NarX and NarQ Sensor-Transmitter Proteins of Escherichia coli Each Require Two Conserved Histidines for Nitrate-Dependent Signal Transduction to NarL. JOURNAL OF BACTERIOLOGY, 177(9), 2416–2424.
Cheung, J., & Hendrickson, W. A. (2009). Structural Analysis of Ligand Stimulation of the Histidine Kinase NarX. Structure, 17(2), 190–201. https://doi.org/10.1016/J.STR.2008.12.013
narX sensor histidine kinase NarX [ Escherichia coli str. K-12 substr. MG1655 ]. (2022, September 22). National Library of Medicine - National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/gene/945788
//cds/membrane/receptor
| activation_coeficient | |
| protein | |
| signalling_molecule |